The possibility has arisen to recover completely from intractable diseases diagnosed as untreatable by conventional medicine. Regenerative medicine using iPS cells is a promising innovation in the medical world. 2014 saw the world's first successful retinal regeneration surgery employing tissue produced from iPS cells. Universities and other research organizations are currently stepping up research activities on regenerative medicine for a variety of ailments. The next challenge in making regenerative medicine widely available is the mass production of target cells of consistent quality from iPS cells. To achieve this, Kyoto University, Sumitomo Dainippon Pharma Co., Ltd., and Hitachi have upped the pace of research & development into automated, mass cell-culturing equipment through open innovation by the three organizations.
In the 10 years since Kyoto University professor Shinya Yamanaka's first production of induced pluripotent stem cells (iPS cells), the medical application of such cells has been progressing. iPS cells are a type of multipotential stem cell that is artificially made from skin and other such types of cells. In addition to the capability of differentiating into all other types of cells, they also retain the significant advantage of being able to multiply virtually without limit through repeated division. If, therefore, cells lost due to illness or injury can be produced based on these iPS cells in the amounts necessary and then transplanted, healing becomes a possibility.
Having passed through the research phase, regenerative medicine using such iPS cells is now entering the phase of practical application. Professor Jun Takahashi of the Kyoto University Center for iPS Cell Research and Application (CiRA) carries out research on using regenerative medicine to treat Parkinson's disease, a neurodegenerative disorder. Parkinson's disease is caused when the neurons that make dopamine, the brain's neurotransmitter, decrease. Conventional therapy for Parkinson's disease has been limited to the treatment of symptoms. While drugs, etc., achieve some symptomatic improvement, a complete cure has not been possible. As Professor Takahashi explains, concerning the progress of research:

"Producing from iPS cells the neurons that create dopamine and then transplanting them into patients' brains has the potential to effect a complete cure. In 2017, when we transplanted neurons made from human iPS cells into monkey models of Parkinson's disease, limb tremors and other such symptoms decreased. On the basis of these research results, human clinical trials*2 are scheduled for 2018. But to do this, we have one more challenge to tackle."
This challenge is the mass culturing of dopamine-producing cells. The number of cells needed for transplantation into humans for therapeutic purposes is in the order of a billion. To culture mass amounts of cells of consistent quality, it is essential to develop a mass-production process fundamentally different from conventional methods, which rely on the manual techniques of skilled technicians.
Research has largely succeeded in establishing the process itself of culturing the cells needed by regenerative medicine from iPS cells. However, to make regenerative medicine available for actual medical scenarios, it is necessary to have a stable supply of cells. To this end, expectations are being placed on automated culturing instead of conventional manual techniques. Up to now, the mainstream method of transplantation has been autologous transplantation, a process in which cells produced from a patient's cells are amplified and processed on an individual basis and then given back to the patient. While this has the advantage of avoiding immunological rejection, it is burdened with a high unit cost of production. Allogeneic transplantation, however, is a process in which iPS cells are produced from healthy donor cells and then stocked for later use. These are then made into cells in accordance with the therapeutic purpose and transplanted. This process significantly shortens manufacturing time and reduces costs.
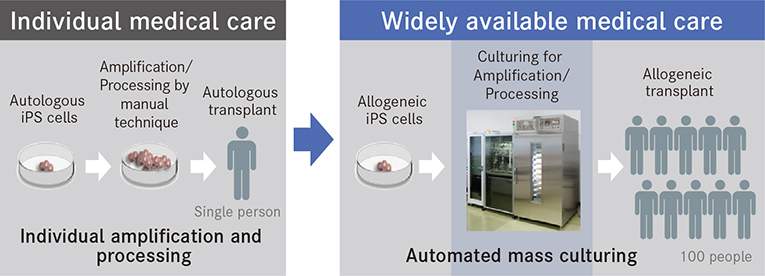
Compared to manual techniques, automated cell-culturing technology can significantly reduce the unit cost of production.
Sumitomo Dainippon Pharma is working on the production of cellular medical agents derived from allogeneic transplantation. According to Dr. Toru Kimura (member of the board of directors, executive officer, senior executive research director in Drug Research Division, and in charge of regenerative & cellular medicine office): "For us, as we aim to supply cellular medical agents for regenerative medicine, there are three challenges. The first is securing quality in mass-produced cells, the second is their stable supply though efficient production, and the third is the reduction of production cost."
Sumitomo Dainippon Pharma has been conducting research & development targeting neurons since the 1990s. From around 2000, the direction of activities settled on nerve regeneration by means of cell transplants. Inspired by Professor Yamanaka's production of iPS cells, the company has been engaged in full-scale operations directed at the regeneration of central nerves using iPS cells. Cellular medical agents produced from iPS cells are now positioned to be one of the core business in 2030.
Dr. Kimura adds, "The development of a dedicated production device is crucial to producing cells of consistent quality. As our partner in this endeavor, we have great expectations for Hitachi and their expertise in closed-system, automated cell-culturing technology. Open innovation—the combining of the scientific knowledge of Kyoto University, Hitachi's superior equipment development engineering, and our drug production technology—has brought our goal within sight."

Center: Jun Takahashi, professor at the Center for iPS Cell Research and Application, Kyoto University. Right: Toru Kimura, member of the board of directors and executive officer, Sumitomo Dainippon Pharma. Left: Shizu Takeda, chief engineer of the Research and Development Group, Hitachi, Ltd.
Hitachi has, from early on, engaged in its own challenge—the development of automated cell-culturing equipment technology. In 2002, Hitachi was approached by Teruo Okano, professor at Tokyo Women's Medical University. Responding to his request for automated cell culturing, our mechatronics team, which specializes in robots, began R&D, eventually moving operations to the Center for Exploratory Research located in Hatoyama-town in Saitama Prefecture, to continue their work.
As Dr. Shizu Takeda, head of the Hitachi Kobe Laboratory, says about what led to development: "Professor Okano wanted the capability to readily bring into the operating room cells contained in a cassette. To enable this, we developed a proprietary, closed-system, automated cell-culturing technology."
The concept behind such a closed-system, automated cell-culturing technology differs fundamentally from conventional manual work or culturing using a robot arm system. With methods like these, the presence of people and robots in what should be a clean room incurs a relatively high risk of contamination from biological or foreign material. The technology developed by Hitachi allows automated culturing of cells to take place in a sterile space that is fully sealed from the outside.
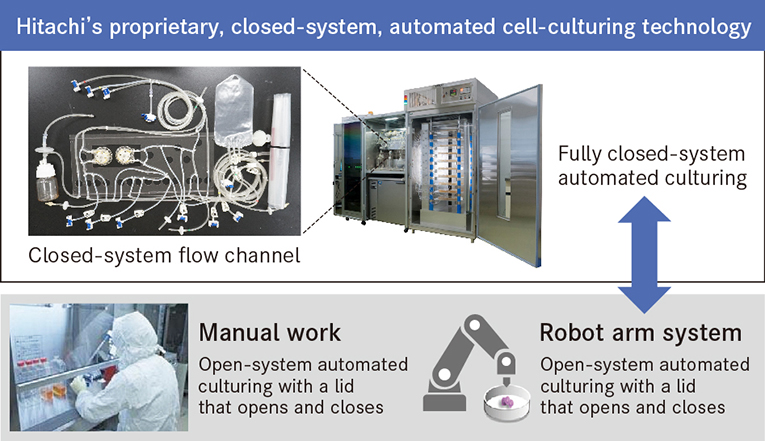
Hitachi's proprietary, closed-system, automated culturing technology allows automated culturing and cell observation in a sterile, closed space.
Professor Takahashi has a high opinion of the automated, mass cell-culturing equipment currently under development based on this technology. He states that, "On top of being able to culture cells in an environment best suited to their growth, we can also obtain highly detailed data in a fully closed environment. I believe there is tremendous significance in making possible that which can't be done manually. This is the decisive difference with the conventional manual technologies that we have simply been trying to automate."
Dr. Kimura discusses his expectations of the automated, mass cell-culturing equipment being jointly developed with Hitachi: "Because it is automatic, this equipment allows for almost no human error to creep in. And because it's a standalone system, it can be expanded as much as necessary anywhere it is needed. We see cellular medicine as one of our core businesses going forward, so when this system is perfected, it promises to be a device we can feel confident about staking our future on."
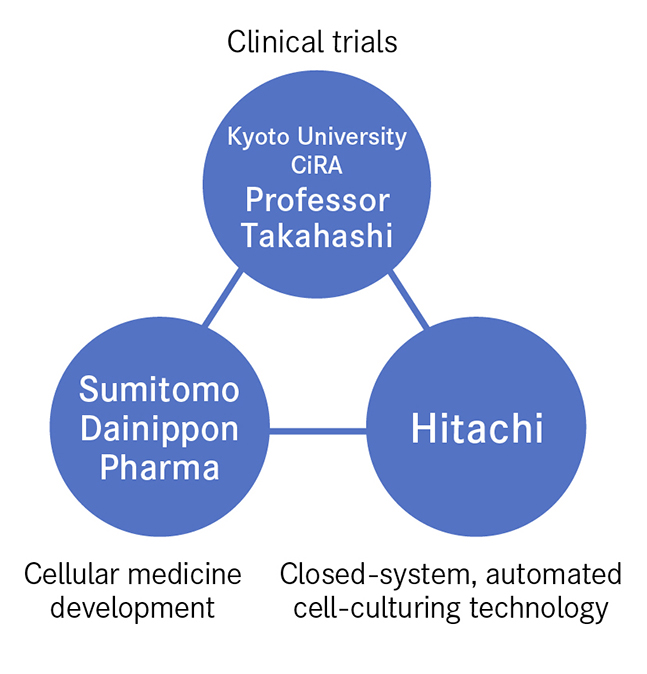
The bringing together of three entities boosted the speed of innovation. These are represented by Professor Takahashi, who established the fundamental technology for cell culturing and carries out clinical trials, Sumitomo Dainippon Pharma, a storehouse of expertise in cellular medicine production, and Hitachi, the source of the closed-system, automated culturing technology needed for mass cell production. Looking back on this process, Professor Takahashi says that "Everyone came together from different places looking to climb to the top of the same mountain. While at the base of the mountain, we had no idea of each other, but as the summit got closer it was only natural that we would meet. From then on, we combined our strengths to attain the summit in one gigantic leap. That's the dynamism I felt."
In 2017, the Hitachi Kobe Laboratory was established at a site about 10 minutes on foot from the Regenerative & Cellular Medicine Kobe Center, Sumitomo Dainippon Pharma's research facility. This move from the Center for Exploratory Research in Hatoyama-town in Saitama Prefecture, engendered some major changes. Dr. Yoshida of Sumitomo Dainippon Pharma puts it this way:
"In the past, we had to change into a suit and take the bullet train all the way into Tokyo for even short meetings. Now, however, all we have to do is take a few steps to meet up right away. The greater degree of communication has clearly changed the atmosphere of the workplace."

From right rear: Kenji Yoshida, Sayaka Sekiya, Atsushi Nakane of the Regenerative & Cellular Medicine Kobe Center, Sumitomo Dainippon Pharma
From left rear: Midori Kato, Hikaru Saito of the Hitachi Kobe Laboratory
As Hitachi equipment was installed in the Sumitomo Dainippon Pharma facility, there are many cases of researchers from both companies conducting experiments in the same room. If a problem arises, they can quickly discuss it on-the-spot, come up with an improvement, and implement it. Research & development made strides in speed as a consequence of going rapidly through the PDCA cycle and getting repeated feedback.
As Dr. Sekiya of Sumitomo Dainippon Pharma comments, "With the same equipment before us, we bounce our opinions off each other, us from our position as manufacturers of cellular medical products and Hitachi from the standpoint of equipment development. Because we talk face-to-face, we can freely speak our minds without formality. We have been able to make a number of useful discoveries because of this. It feels as if the results of a single experiment multiply several times over."
Hitachi's response has also sped up. Ms. Kato, researcher at the Hitachi Kobe Laboratory, provides some details:
"Anything brought up during daytime meetings we share with the person in charge of the equipment before the day is out, and then, on the next day, we implement improvements, which we then have checked by Sumitomo Dainippon Pharma's person in charge. You can tell that we are making steady progress forward, which motivates me more as an engineer."

Research activity at the Hitachi Kobe Laboratory

Video: Hitachi Kobe Laboratory — Initiatives for Regenerative Medicine
Professor Takahashi evaluates the efforts of the three entities as follows: "I felt strongly the significance of combining strengths from different standpoints while heading toward the same goal from the earliest stages. The direct bouncing of ideas off each other during the course of our research has produced a unique dynamism. It's nothing like when I was in academia and was closed off from the outside world. There is a real sense of research intensification."
To develop automated, mass cell-culturing equipment, Hitachi put together a team of people having different areas of expertise—from physics, to electrical circuits, cells, and so forth—to take charge of their respective fields. This is a Hitachi strength; that is, researchers and engineers from different backgrounds can gather as necessary into a single team at any time. So, for the development of the cell quality inspection equipment and production information management systems that are next on the schedule, we are likely to see new experts added to the team. We may also see a future need for analysis technology using Hitachi's proprietary artificial intelligence (AI).
Expressing her hopes, Dr. Takeda says that "Our mission is to provide comprehensive solutions in the field of regenerative medicine. But we are not content to stop there. We also have our eyes fixed on contributing to the pharmaceuticals field."
Patients suffering intractable diseases are not limited to Japan. They can be found everywhere around the world. Thus, concludes Dr. Kimura, "We are also going to leverage Hitachi's global network to make made-in-Japan regenerative medicine globally available. The open innovation prompted by our industry-academia partnership, one that goes so far as to embrace this goal, too, may very well become a new model for future research & development."
Release Date: August 2018
Solutions By: Research & Development Group, Hitachi, Ltd.







